Total Synthesis of Bactobolin A (J. Švenda, 2020)
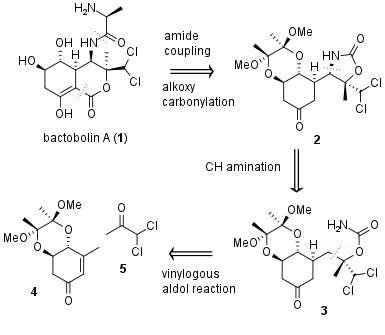
Bactobolin A (1) is a complex polyketide firstly isolated in 1979 by S. Kondo et al. from bacteria (Pseudoinonas BMG13-A7). More than ten years later R. S. Garigipati and S. M. Weinreb successfully described the first total synthesis. Now in 2020, the research group of Jakub Švenda from Masaryk University (Brno, Czech Republic) published a new asymmetric total synthesis in 10% overall yield from commercially available starting materials in JACS. From a retrosynthetic view (see Figure 1) the alanine moiety was introduced in a late-stage peptide coupling. Furthermore, alkoxy carbonylation should lead to C-C bond formation and cyclization. The precursor 2 was synthesized by CH amination from 3, which itself is derived from quinic acid derivative 4 and ketone 5 by vinylogous aldol addition.
Figure 1: Retrosynthetic analysis of bactobolin A (1).
Synthesis of Aldol Precursor
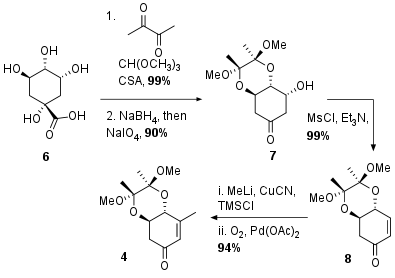
The synthesis of aldol precursor 4 is adapted from previous synthetic efforts to quinic acid derivatives. As shown in Scheme 1 Quinic acid (6) was transformed into ketone 7 by selective protection of the diol, NaBH4 reduction of the acid and periodate cleavage to 7 in 90% yield of two steps. The following elimination led to unsaturated ketone 8 in again excellent yield. After Michael addition of the in situ generated cuprate, the enol was trapped as silyl enol ether and subsequent Saegusa Ito oxidation liberated 4 in 94% yield.
Scheme 1: Synthesis of precursor for aldol reaction from quinic acid (6).
Vinylogous Aldol Reaction
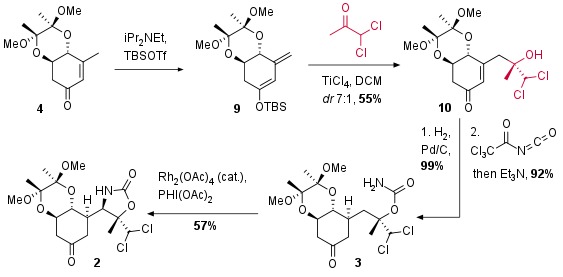
For the following vinylogous Mukaiyama type aldol reaction substrate 4 was transformed into silyl enol ether 9 (see Scheme 2). During the formation of 9 also deprotonation in alpha position of the ketone occurred in a ration of 10:1 depending on the type of base (not shown). Lewis acidic activation then enabled the vinylogous aldol addition in a good diastereomeric ratio (7:1). The authors describe lower ratios using other Lewis acids and diol protecting groups. The reduction of the double bond under common conditions (99%, dr 15:1) was followed by the introduction of the carbamate moiety of 3. Rhodium catalyzed CH amination finally led to bicyclus 2 in moderate yield as a single isomer.
Scheme 2: Vinylogous aldol reaction and CH amination as key steps of the total synthesis.
Completion of Total Synthesis
The following intramolecular alkoxycarbonylation was already explored during the first total synthesis in 1988 by Weinreb et al. In this total synthesis, the authors decided to activate the oxazolidinone by the introduction of a 2-nitrobenzenesulfonyl group. An excess of sodium hydride led to the enolization of the ketone and attack at the oxazolidinone carbonyl center. Direct deprotection of 11 liberated bicyclic 12 in 62% yield over 2 steps (see Scheme 3). To complete the total synthesis alanine coupling to 13 in 73% yield was followed by acidic deprotection to bactobolin A (1) in 92% yield.
Scheme 3: Completion of the total synthesis of bactobolin A (1).
Published in P. Vojáčková, L. Michalska, M. Nečas, D. Shcherbakov, E. C. Böttger, J. Šponer, J. E. Šponer, J. Švenda J. Am. Chem. Soc. 2020, 142, 7306-7311. doi: 10.1021/jacs.0c01554


No Comments