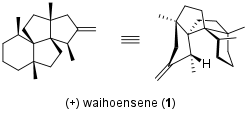
First Asymmetric Total Synthesis of Waihoensene (Z. Yang, 2020)
Although isolated already in 1997, the complex diterpene waihoensene was firstly synthesized twenty years later as a racemic mixture. In 2020 the Zhen Yang research group from Peking University (State Key Laboratory of Chemical Genomics based in Shenzhen) published the first asymmetric version in JACS.
 In the beginning starting material 2 was treated with Grignard reagent to achieve 3 in excellent yield (see Scheme 1). The following chiral 1,4-addition set the first quaternary stereocenter in good enantioselectivity. Trapping of the enolate and oxidation led to alkenoate 4 in 61% yield. The following Sakurai reaction with allyl TMS delivered 5 as a diastereomeric mixture. The following ozonolysis yielded into the aldehyde which was transformed into the terminal alkyne 6 by reaction with the Ohira Bestmann reagent. After the formation of the enolate 7 5-exo-dig cyclization led to bicyclus 8.
In the beginning starting material 2 was treated with Grignard reagent to achieve 3 in excellent yield (see Scheme 1). The following chiral 1,4-addition set the first quaternary stereocenter in good enantioselectivity. Trapping of the enolate and oxidation led to alkenoate 4 in 61% yield. The following Sakurai reaction with allyl TMS delivered 5 as a diastereomeric mixture. The following ozonolysis yielded into the aldehyde which was transformed into the terminal alkyne 6 by reaction with the Ohira Bestmann reagent. After the formation of the enolate 7 5-exo-dig cyclization led to bicyclus 8.
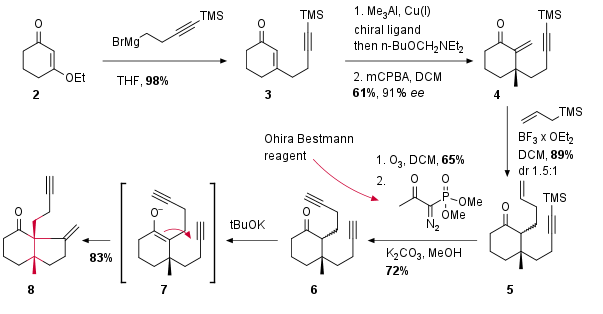 Scheme 1: Synthesis of fragment 8 through cyclization.
Scheme 1: Synthesis of fragment 8 through cyclization.
With bicyclus 8 in hand, the following Pauson Khand cyclization built up the final tetracyclic ring system as shown in Scheme 2. Under optimized conditions, 9 could be synthesized in 59% yield. After methylation to 10 in 81% yield, the ketone attached on the cyclohexane was transformed to the terminal olefine in a three-step procedure including a protection/deprotection sequence of the competing, second ketone. Stereoselective reduction of 11 was achieved using Hydrogen Atom Transfer (HAT). In this radical reaction hydrogen from the alpha position of the ketone was transferred to the olefine in a [1,4] or [1,5] HAT process which led to only one diastereomer through substrate control. Other reduction protocols failed to deliver the desired stereoisomer. Finally, 12 was regioselective methylated in alpha position followed by olefination to the natural product 1.
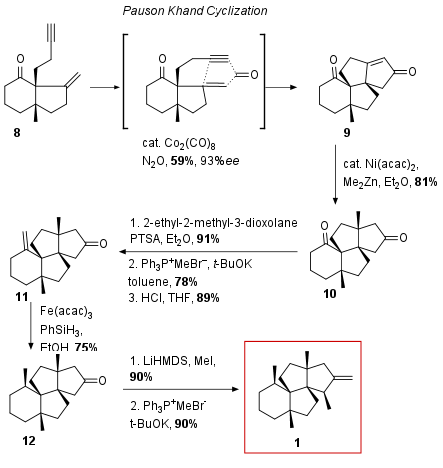 Scheme 2: Completion of total synthesis of 1.
Scheme 2: Completion of total synthesis of 1.
This first asymmetric total synthesis impressed by its short route (15 steps) and fast assembling of a highly complex tetracyclic ring system. Also the stereoselective HAT could be interesting for other purposes when common reduction methods fail.
Published by Y. Qu, Z. Wang, Z. Zhang, W. Zhang, J. Huang, Z. Yang J. Am. Chem. Soc. 2020, 142, 6511-6515. doi: 10.1021/jacs.0c02143
