Bioinspired Total Synthesis of Brevianamide (A. Lawrence, 2020)
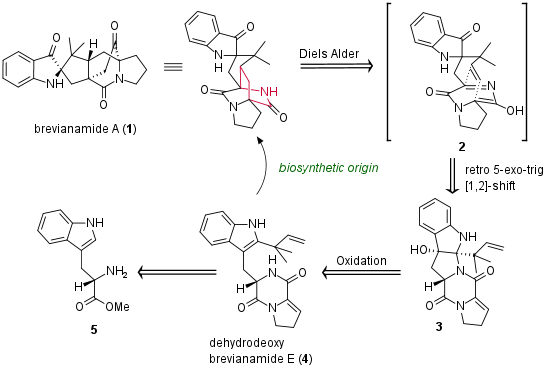
Although already isolated by Birch et al. in 1969, it took 50 years for the first total synthesis of brevianamide A (1). Published in Nature Chemistry from the research group of Andrew Lawrence (University of Edingburgh, Scotland, UK), the authors not only present a synthetic access, they also make a new proposal for the biosynthetic origin of this complex alkaloid. As shown in Figure 1, they envisioned that the oxidation of the natural occuring dehydroxydeoxy-brevianamide E (4) could deliver 3. This intermediate then should undergo a retro 5-exo-trig cyclixation and a [1,2] alkyl shift yielding in Diels Alder precursor 2.
Figure 1: Retrosynthetic analysis of the total synthesis of brevianamide (1).
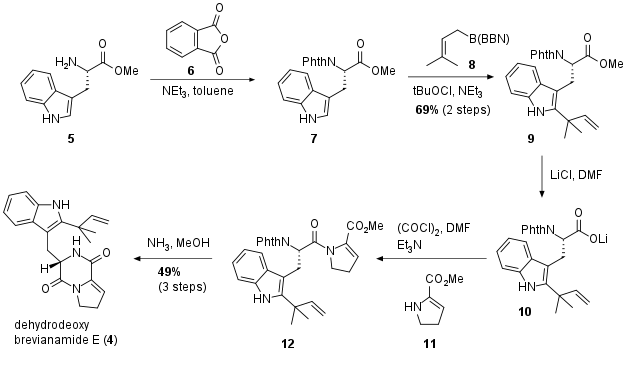
To test their hypothesis they started to synthesize 4 out of commercially available tryptophan methyl ester 5 (see Scheme 1). After protection of the primary amine with 6, indole 7 was subjected to reverse prenylation conditions with 8 yielding in 9 in 69% yield over two steps. The following unusual saponification prevented the partial deprotection of the amine and delivered 10 which was directly transformed into the acid chloride and afterwards coupled with amine 11. Finally, 12 was deprotected to 4. Note that the authors were able to synthesize this precursor in only 5 steps (34% yield.)
Scheme 1: Synthesis of dehydrodeoxy brevianamide E (4).
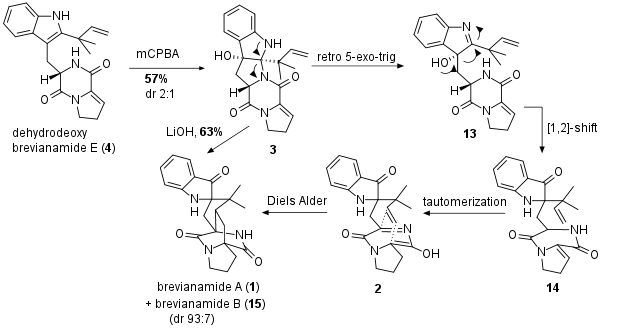
With 4 in hand, the oxidation delivered 3 in 57% yield as shown in Scheme 2. The diastereomeric ratio remained poor, however the other diasteromer (not shown) was successfully transformed into (ent)-brevianamide A. In the last step 3 was treatened with LiOH to delivier 1 together with the diasteromer brevianamide B (15) in 63% yield (dr 93:7, ee 93:7, 99:1 after recrystallization). The authors propose a mechanism in which 3 opens in a retro 5-exo-trig to 13 followed by a [1,2]-alkyl shift to 14 and finally tautomerization to 2. Spontaneous Diels Alder reaction then delivers 1. The diasteromeric ratio between brevianamide A and B are close to the natural occuring ratio during the isolation of the natural products. The authors therefore propose a spontaneous Diels Alder reaction during biosynthesis without enzyme incooperation.
Scheme 2: Completion of total synthesis of brevianamide A (1) and brevianamide B (15).
Published by R. C. Godfrey, N. J. Green, G. S. Nichol and A. L. Lawrence in Nature Chemistry 2020. https://doi.org/10.1038/s41557-020-0442-3

No Comments